The high standards of NF T 72-281
The NF T 72-281 standard is a protocol designed by the French standardisation body AFNOR. It is designed for airborne surface disinfection systems, and it is recognized for its particularly strict fulfilment conditions. NF T 72-281 defines a set of testing methods to challenge disinfectant products in real-life conditions; efficacy data is obtained after tests conducted only according to the intended manner of use. When considering any airborne disinfectant instrument, it is important to verify compliance with NF T 72-281
NF T vs existing EN
AFNOR published the first version of NF T 72-281 in 1980, and has updated it a number of times since, with the latest iteration being in 2014 (NF T 27-281:2014). The necessity for this standard lies in the unsuitability of existing standardised test methods for biocidal materials delivered via air. Current European standards (EN) testing for the efficacy of disinfecting agents only test by direct application methods; meaning the allegedly biocidal product is in liquid form, and either submerged or directly entering in contact with target microorganisms. Those types of testing are usually considered to be of type “Phase 1”; and while they are a good indicators of the biocidal nature of the tested substances, Phase 1 tests are not representative of the real-life usage of biocidal agents. This is a particularly important distinction in the case of airborne disinfectants, where active agents are delivered as gas, vapour, mist or fog, and have completely different parameters of action (concentration, surface contact and time).
Because regulatory bodies are aware of this issue, they currently use NF T 72-281 as the starting point for a new EN standard dedicated to airborne disinfection systems (prEN 17272). In the meanwhile, the Biocidal Products Regulation (BPR, Regulation (EU) 528/2012) only accepts the NF T 72-281 standard for airborne surface disinfection systems. So when choosing an airborne disinfecting instrument, it is highly recommended to select a product that uses the NF T 72-281 standard for its efficacy claims. Manufacturers of airborne disinfecting materials that base their efficacy claims on unsuitable EN standards provide inapplicable figures, which can potentially put a health threat to the user and the environment.
NF T tests and settings
NF T 72-281 is considered a “Phase 2, Step 2” assay conducted in a semi-field method. This means the disinfecting agent is tested as intended to use, and target microorganisms that are placed on a representative surface. Moreover, the test is carried out in a standardized test room, and the target samples are placed at a distance, facing away from the source of the disinfecting agent; for example, an ozone generator.
As for all microbiology tests, NF T 72-281 measures efficacy against a logarithmic scale. Microorganisms are counted as numbers of colony forming units (CFUs), and efficacy is given as the difference between the CFU count before and after application of the disinfecting agent. The result is given as a number of “log-reduction”, where a log-reduction of 1 corresponds to a 10-fold reduction. For example, for a 106 initial CFU, a log-reduction of 4 would see a reduction to 102 CFU after treatment. This is typically marked on commercial packaging as a kill-rate percentage, where a log-2 reduction corresponds to a 99% germicidal power, a log-3 to 99.9%, and so forth.
NF T 72-281 is defined as a methodology for the “determination of bactericidal, fongicidal, yeasticidal, mycobactericidal, tuberculicidal sporicidal and virucidal activity, including bacteriophages”. The biocidal efficacy requirements depend on the target organisms:
- Bacteria: > 5-log reduction
- Spores: > 3-log reduction
- Fungi & yeasts: > 4-log reduction
- Viruses incl. phages: > 4-log reduction
- Mycobacteria: > 4-log reduction
Initial conditions require 1-log above the log-reduction objective (e.g., for bacteria, a load of 106 CFUs must be applied to the target surface).
Test microorganisms
Manufacturers are required to perform their efficacy tests on microorganism strains pre-approved by the NF T 72-281 standard. Those strains are representatives of the different target types (bacteria, spores, viruses, bacteriophages, mycobacteria, fungi and yeasts). Occasionally, more than one strain for a single target type is required, notably when variants exist (e.g. P. aeruginosa, S. aureus, E. hirae and E. Coli for bacteria, covering Gram-positive and Gram-negative strains).
Depending on the intended field of application of their disinfectant products, manufacturers can perform their tests on the entirety or part of those reference organisms. If additional target microorganisms are added to the methodology, proper documentation has to be provided covering the reason for their addition, the conditions at which they were grown and stored, and their general suitability for the NF T 72-281 standard. In addition to the entirety of the test microorganisms listed in NF T 72-281, STERISAFE also tests for additional target pathogens, selected for their relevance in particular applications (e.g. Vancomycin-resistant enterococci, VRE, for hospital disinfections). For the complete list of tested microorganisms, please refer to the Table in annex of the present document.
STERISAFE’s vision
STERISAFE uses the NF T 72-281 standard for all its products, and as such can guarantee their efficacy. Considering the test conditions, the high kill-level objectives it requires, and its large target range, the NF T 72-281 should be the only acceptable standard methodology for efficacy claims of airborne disinfectant products. STERISAFE has been a pioneer in using this standard in the industry of the ozone-based disinfection systems, and will continue to do so.
Addendum (November 2020)
As of October 2020, EN 17272:2020 (Methods of airborne room disinfection by automated process) repelled NF T 72-281 and was given the status of national standard across the European Union. In effect, this mean that while older NF T 72-281 results can still be accepted by competent authorities, efficacy tests done after October 2020 have to follow the new norm; and that manufacturers should actively seek to re-run their efficacy tests under EN 17272 conditions.
As the new European standard is heavily based on the older French norm, the differences between the two protocols are few, but important (listed here are the most important items from a STERISAFE PRO perspective):
- Addition of a “distribution test”, designed to ensure efficacy of the process throughout the room by placing samples at four distinct positions;
- Substitution of skimmed milk by bovine serum albumin (BSA) as the main interfering substance in clean conditions;
- Addition of test microorganisms ( baumanii, P. hauseri);
- Modification of the required test microorganisms list corresponding to different fields of application;
- For applications in the medical area, the efficacy requirement for spores is now at > 4-log reduction.
Following STERISAFE’s policy of always adhering to the strictest existing standards, the complete overhaul of the efficacy tests related to the STERISAFE PRO is currently ongoing, with the objective to be fully EN 17272- compliant in the near future.
Reference
- L’Association Française de Normalisation (2014). NF T 72-281. Procédés de désinfection des surfaces par voie aérienne – détermination de l’activité bactéricide, fongicide, levuricide, mycobactéricide, tuberculocide sporicide et virucide incluant les bactériophages. Paris: AFNOR
- European Committee for Standardization (2020). EN 17272. Chemical disinfectants and antiseptics – Methods of airborne disinfection by automated process – Determination of bactericidal, mycobactericidal, sporicidal, fungicidal, yeasticidal, virucidal and phagocidal activities. Brussels: CEN
STERISAFE’s NF T 72-281 results
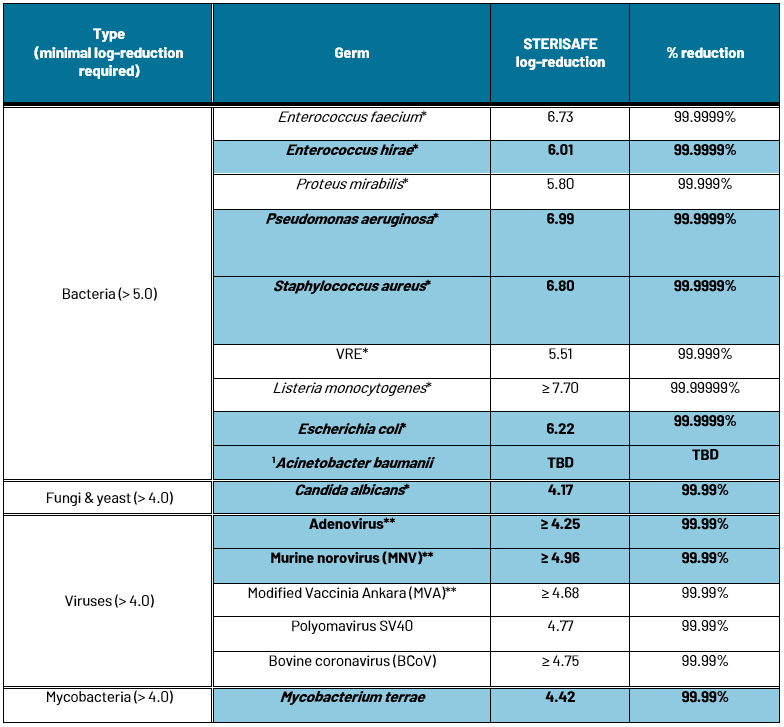
In blue: Reference microorganisms listed in NF T 72-281
* Tested by Danish Technological Institute
** Tested by Dr. Brill + Partner GmbH Institute for Hygiene and Microbiology
*** Tested by INFUSER ApS and Metropolitan University College
